伊人直播
Natural products have always been an important resource for drug discovery and development due to their structural uniqueness and diversity, and more than half of the small molecule drugs are related to natural products. However, the structural complexity of natural products and the limitations of their sources have largely restricted their further development.Therefore the development of novel methods for the synthesis of natural products is essential.
On June 25, 2024, the research group led by Professor Xiaoguang Lei from the College of Chemistry and Molecular Engineering at Peking University published a research paper entitled ‘Chemoenzymatic total synthesis of alchivemycin A’ in Nature Synthesis. The chemoenzymatic strategy was used in this research to address the challenges associated with total synthesis of alchivemycin A.
Alchivemycin A belongs to a unique class of polyketide natural products isolated from plant-derived actinomycete Streptomyces. It shows potent antibacterial activity and anti-tumour activity.Alchivemycin A features a unique 2H-tetrahydro-4,6-dioxo-1,2-oxazine (TDO) ringsystem, an unprecedented structural motif in reported natural products. It also has a 17-membered polyhydroxy macrocyclic ring, a highly functionalized cis-decalin fragment and two epoxy groups. Its inherent structural complexity and high oxidation statepresent enormous synthetic challenges (Fig.1).
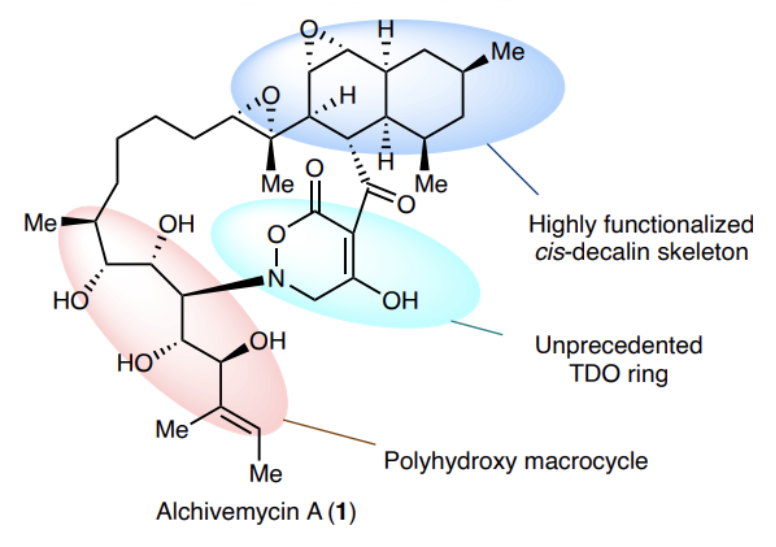
Fig.1 The structural features and complexity of alchivemycin A present enormous synthetic challenges.
Compared with traditional chemical synthesis, enzyme catalysis has unique potential to simplify the synthesis of complex natural products. The chemoenzymatic strategy, which combines enzymatic catalysis with chemical methods, is increasingly applied. Insights from natural product biosynthesis may inspire synthetic chemists to devise new strategies for addressing the challenges of complex natural product synthesis.
Inspired by the biosynthetic pathway of alchivemycin A reported by Prof. Huiming Ge's group at Nanjing University, Lei's group presents here the total synthesis of alchivemycin A using a chemoenzymatic approach that combines de novo skeleton construction and late-stage enzymatic oxidation reactions. The convergent synthesis of the highly functionalized unnatural tetramic acid-bearing intermediate was primarily achieved through boron-alkyl Suzuki–Miyaura cross-coupling, macrolactamization and Lacey–Dieckmann condensation reactions. Subsequent late-stage enzymatic epoxidations of the unnatural substrate with AvmO3 and AvmO2 yielded the corresponding epoxides regio- and stereoselectively in high yields. Finally, the variant AvmO1-Y282R developed via rational protein engineering was used to establish the TDO ring system, resulting in the synthesis of alchivemycin A (Fig.2). Importantly, no currently available chemical methods could directly convert the tetramic acid ring into the TDO ring. The TDO ring is crucial for the biological activity of alchivemycin A and represents a novel heterocyclic structure for medicinal chemistry. This work highlights the efficacy of late-stage enzymatic oxidation in the efficient synthesis of complex natural products in a highly oxidized state. This work also paves the way to develop a chemical probe based on alchivemycin A to investigate its antibacterial and anticancer mechanisms of action and to identify new cellular targets for drug discovery.
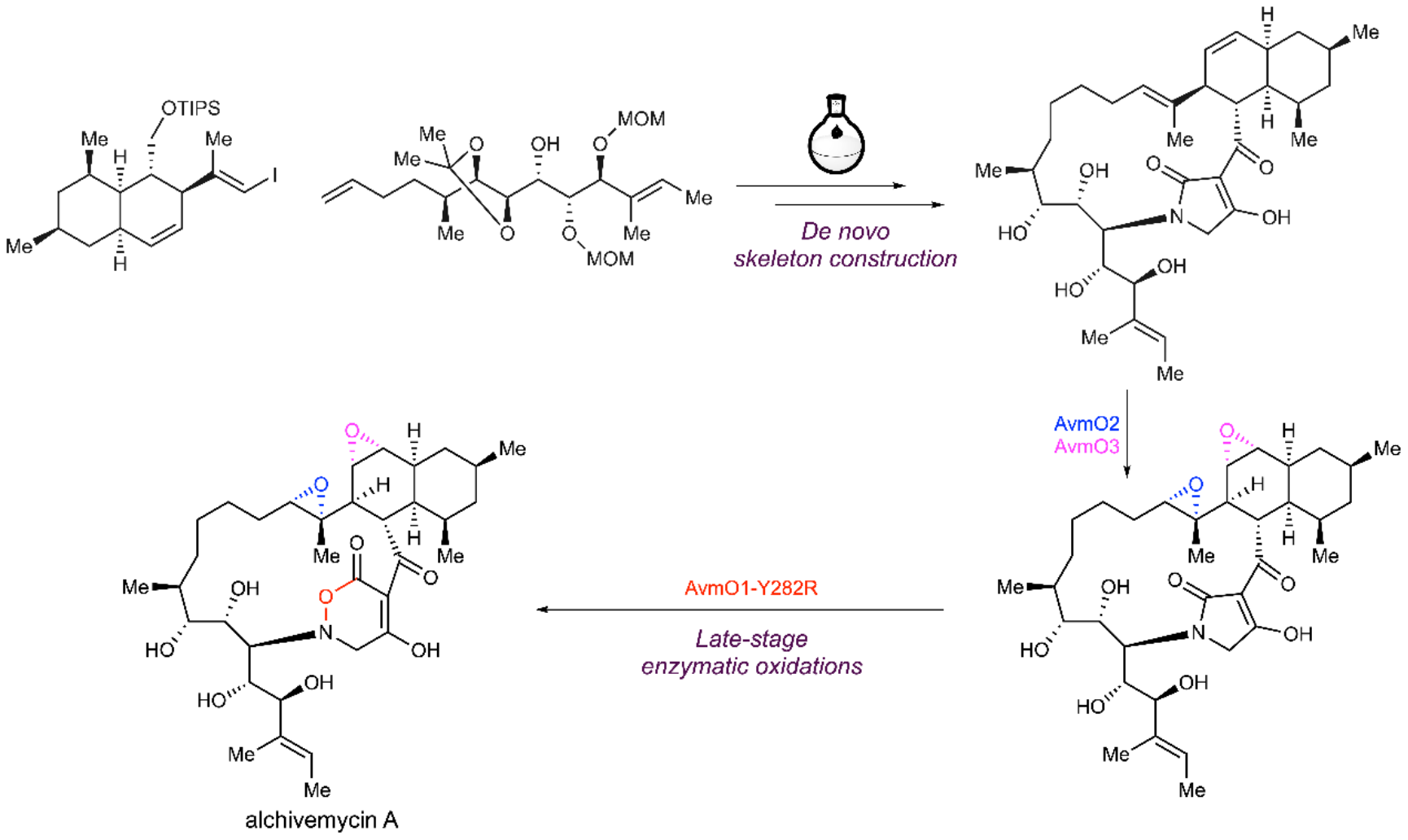
Fig.2 Total synthesis of alchivemycin A using a chemoenzymatic approach.
Haoran Dong and Nianxin Guo (both Ph.D. candidates) from the College of Chemistry and Molecular Engineering, Peking University are co-first authors of the article. Prof. Xiaoguang Lei from the College of Chemistry and Molecular Engineering at Peking University is the corresponding author. The research was supported by the National Key Research and Development Plan, the National Natural Science Foundation of China and the Beijing National Laboratory for Molecular Sciences. A special research grant for biocatalyst development from Novartis Pharma is acknowledged. X.L. is supported by the New Cornerstone Science Foundation through the XPLORER PRIZE.
Link to the original article://www.nature.com/articles/s44160-024-00577-7