伊人直播
The teams led by Prof.Chu Wang at the College of Chemistry and Molecular Engineering,Peking University and Prof. Junyu Xiao at the School of Life Sciences, Peking University have developed a chemical proteomics method named “METAL-TPP” to systematically identify metal binding proteins in proteomes. It provides a new experimental technique for studying metal-binding proteomics. The research results were recently published online in Nature Chemical Biology with the title "Discovery of metal-binding proteins by thermal proteome profiling".
Metal-binding proteins are widely distributed in living organisms and play crucial roles in many important biological processes, such as catalysis, transcription, and signal transduction. Moreover, an increasing amount of research has found a close relationship between many human diseases and the abnormal function of metal-binding proteins. Currently, methods available for identifying metal-binding proteins at the proteome level are limited, and come with certain drawbacks in terms of universality, sensitivity, and accuracy. Therefore, the development of systematic chemical proteomic methods for identifying metal-binding proteins is of particular importance for studying the biological functions of these proteins. This advancement can contribute to better comprehension of the significant roles played by metal-binding proteins in both physiological and pathological processes.
The Thermal Proteome Profiling (TPP) method enables unbiased identification of drug targets in living cells at the proteome level. Furthermore, many studies have revealed that the binding of metal ion can enhance the thermal stability of metal-binding proteins. Inspired by these findings, the authors investigated the changes in the thermal stability of metal-binding proteins after metal ions of the proteins were chelated by metal chelators. They developed a novel method, named "METAL-TPP" (Metal Extraction Triggered Agitation Logged by Thermal Proteome Profiling), based on the changes inthermal stability to discover metal-binding proteins.
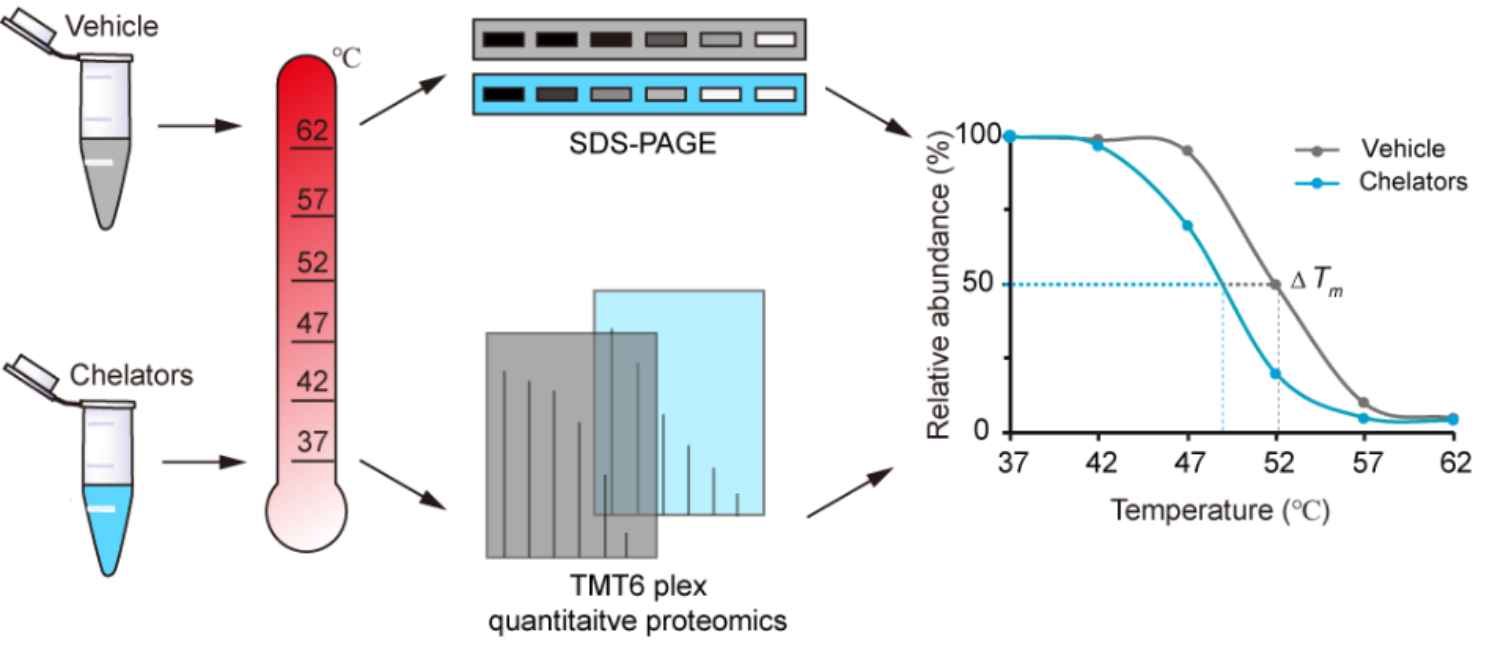
Fig.1 Scheme of METAL-TPP
The authors firstly demonstrated that METAL-TPP can effectively detect the decrease in thermal stability of metal-binding proteins caused by the broad-spectrum metal chelator, EDTA, at the level of purified proteins and cell lysates. Subsequently, theyfound that among the 125 proteins with reduced thermal stability, 102 (82%) were known metal-binding proteins, and 23(18%) were unnoted as metal-binding proteins which are potential metal-binding proteins.
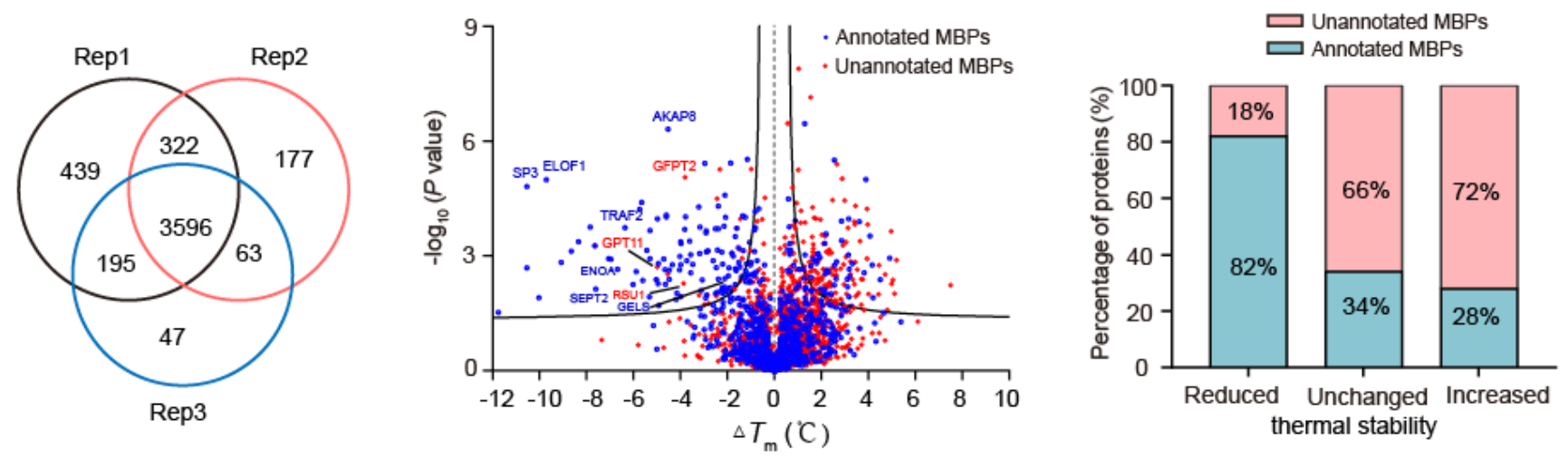
Fig.2 Results of METAL-TPP using EDTA
Among these potential metal-binding proteins, the biologically significant protein GFPT2 was chosen for further biochemical validation. GFPT1/2 is the rate-limiting enzyme in the first step of the hexosamine-biosynthetic pathway. UDP-GlcNAc is the final product of this pathway. The authors demonstrated that zinc ions can interact with GFPT2, and inhibit the protein's activity at the cellular level. Moreover, the presence of zinc ions significantly decreases the levels of UDP-GlcNAc, indicating that zinc ions can regulate the hexosamine-biosynthetic pathway by inhibiting the activity of GFPT2. Interestingly, zinc ions exhibit different selectivity in inhibiting the activities of GFPT2 and GFPT1, suggesting a novel regulatory mechanism.
The authors purified the orthologue protein of GFPT2, GLMS, in E. coli. Biochemical experiments and crystal structure analysis revealed that GLMS is a zinc-binding protein, with zinc ions bound near the substrate-binding region. This implies that zinc ions may inhibit the enzyme activities of GLMS and GFPT2 by competing with substrates and coordinating at catalytic sites.
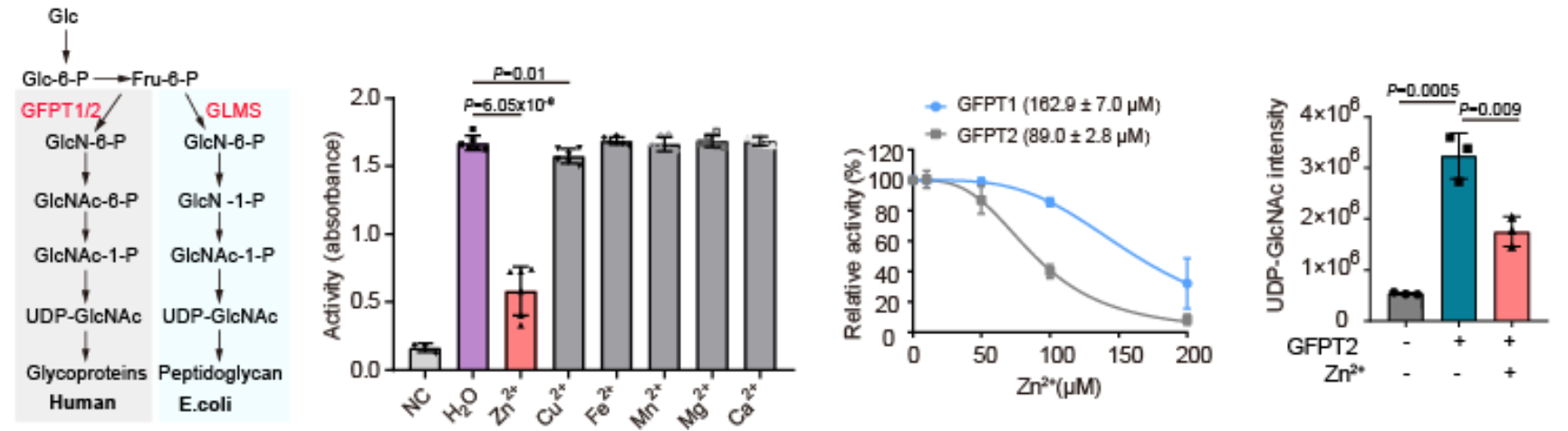
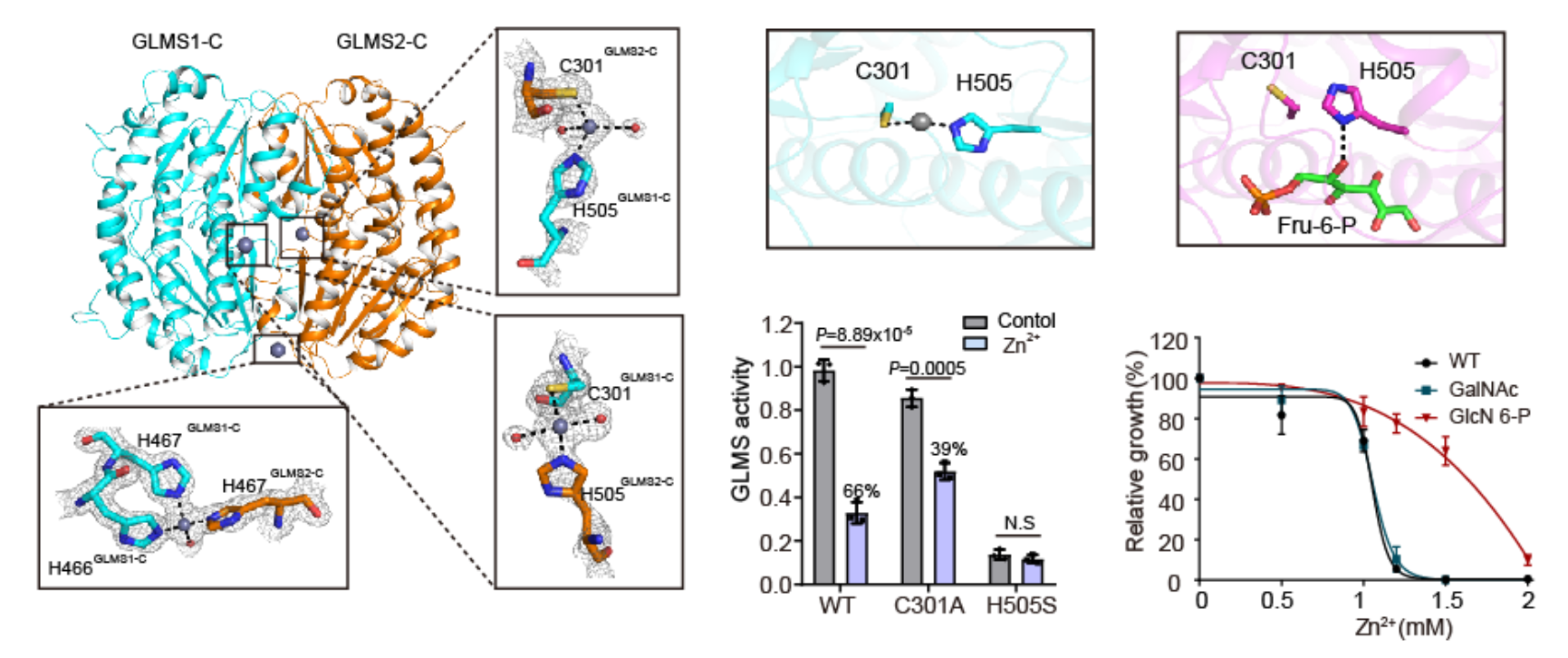
Fig.3 Zinc binds and inhibits GFPT/GLMS enzymes
Finally, the authors expanded the applicability of METAL-TPP to identify the metal-binding proteins using another metal chelating agent, TPEN. They found that 110 (73%) of the proteins with decreased thermal stability were known metal-binding proteins, indicating that TPEN can specifically identify metal-binding proteins similar to EDTA. Among these, 95 (86%) of the known metal-binding proteins were zinc-binding proteins. While 41% of the proteins with decreased thermal stability induced by EDTA were zinc-binding proteins, suggesting a certain preference of TPEN for identifying zinc ion-binding proteins.
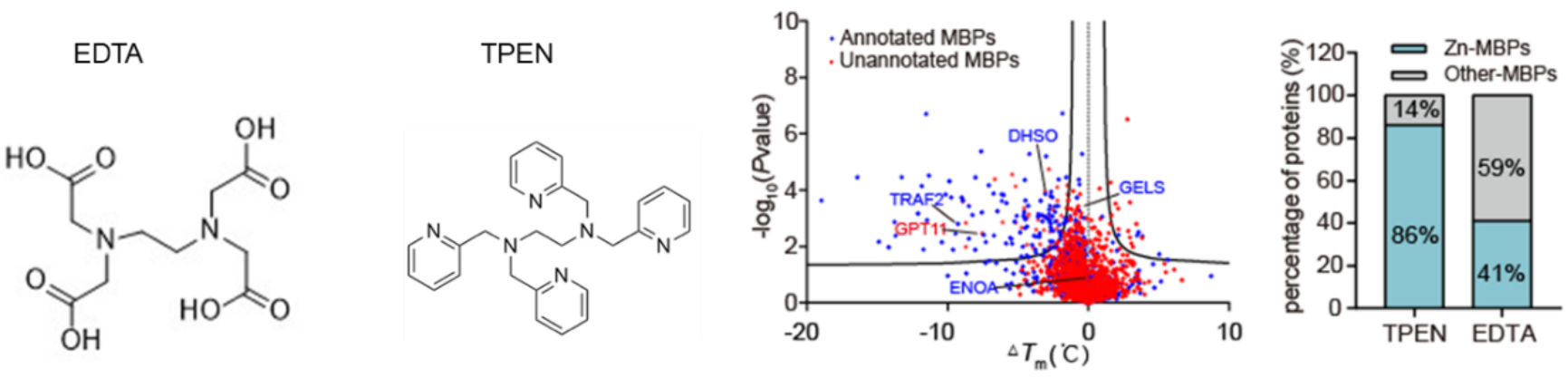
Fig.4 Results of METAL-TPP using TPEN
In summary, the authors have introduced a novel method, METAL-TPP, for systematically identifying metal-binding proteins at the proteome level. Through perturbing metal-binding proteins with two different types of metal chelating agents and examiningthe changes in thermal stability, this study has revealed a series of potential metal-binding proteins. This work will provide a valuable database for further investigation into the biochemical functions of metal-binding proteins. And it contributes to further research on the role of metal-binding proteins in diseases.
Prof. Chu Wang and Prof. Junyu Xiao are the co-corresponding authors of this paper. Dr. Xin Zeng, Dr. Tiantian Wei from Peking-Tsinghua Center for Life Sciences, Mr. Xianghe Wang and Dr. Yuan Liu at College of Chemistry and Molecular Engineering, Peking University, are the co-first authors of this paper. Other collaborators involved in this research are Zhen-Shu Tan, Yihai Zhang, Tanuyu Feng, Dr. Yao Cheng, Fengzhang Wang, Bin Ma, Prof. Wei Qin, and Dr. Chuanping Gao. This work was supported by the National Natural Science Foundation of China.Prof. Chu Wang acknowledges the support from Synthetic and Functional Biomolecules Center, Beijing National Laboratory for Molecular Sciences, Key Laboratory of Bioorganic Chemistry and Molecular Engineering of Ministry of Education, College of Chemistry and Molecular Engineering, Peking-Tsinghua Center for Life Sciences, Academy for Advanced Interdisciplinary Studies at Peking University. Prof. Jun-Yu Xiao acknowledges the support from State Key Laboratory of Protein and Plant Gene Research, School of Life Sciences, Peking-Tsinghua Center for Life Sciences, Academy for Advanced Interdisciplinary Studies at Peking University.
Original link for the paper: //www.nature.com/articles/s41589-024-01563-y