伊人直播
As natural biomacromolecules, proteins possess immense clinical potential as therapeutic agents for various diseases, including cancer, immune disorders, and metabolic abnormalities. Compared to small-molecule drugs, therapeutic proteins offer advantages such as programmability, multifunctionality, high specificity, low toxicity, and superior efficacy. However, their clinical application is often hindered by issues like poor stability and short in vivo half-lives. Therefore, therapeutic proteins are typically bioconjugated with half-life extension modules to optimize their therapeutic window and improve treatment outcomes. Chemical topology provides a unique dimension for the design and modification of protein bioconjugates, which has increasingly attracted the attention of researchers. Several studies have shown that topological engineering of protein-based bioconjugate drugs can significantly improve their thermal stability, in vivo half-lives, and antitumor efficacy.
Recently, the research group led by Professor Wen-Bin Zhang from the College of Chemistry and Molecular Engineering at Peking University published a research paper entitled "Probing the Topological Effects on Stability Enhancement and Therapeutic Performance of Protein Bioconjugates: Tadpole, Macrocycle versus Figure-of-Eight" in Advanced Healthcare Materials. The study reports the design, synthesis, and characterization of therapeutic protein bioconjugates in three topologies (i.e., tadpole, macrocycle, and figure-of-eight), and discusses the subtle influence of topology on protein drug performance.
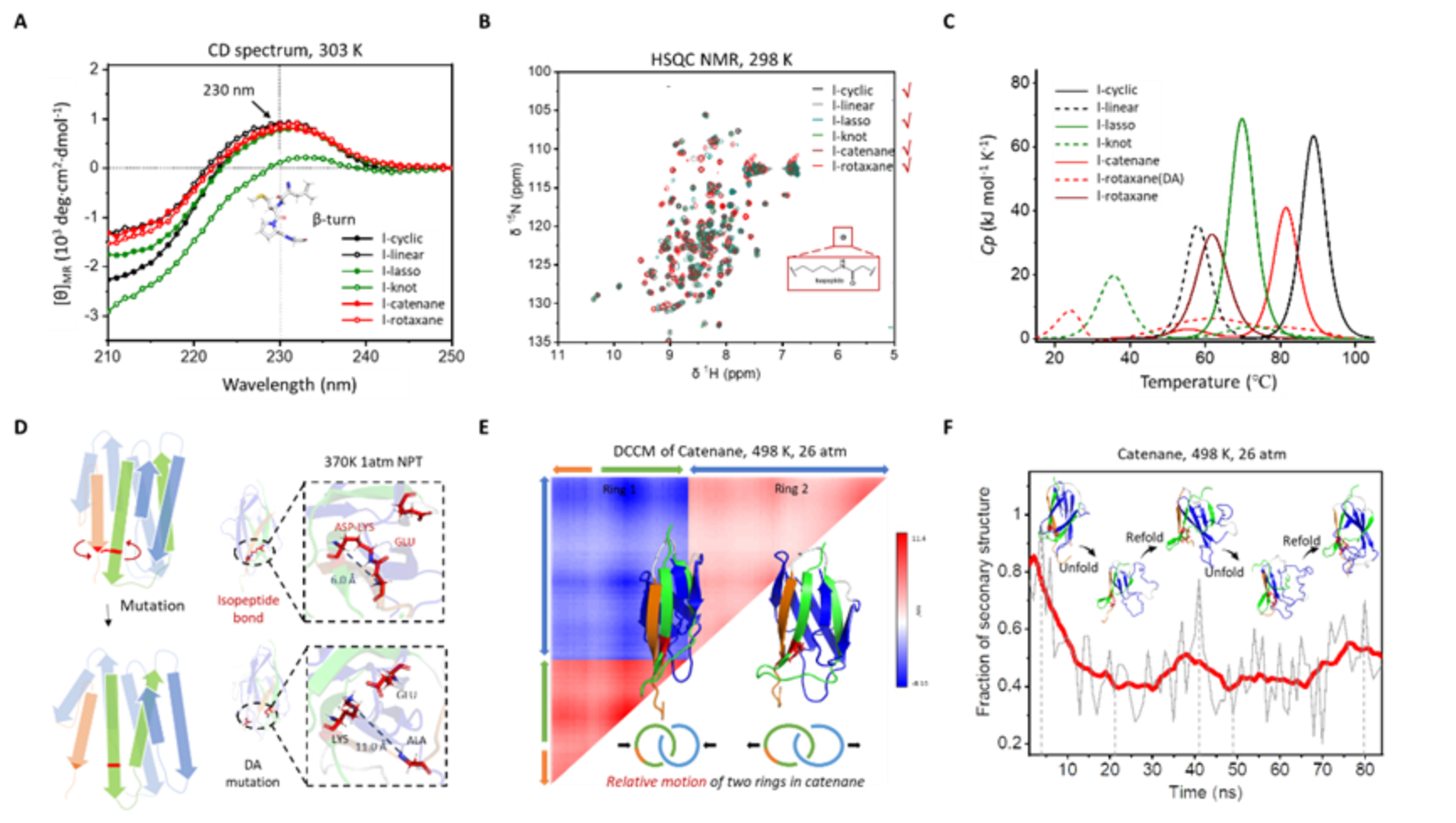
The interferon α2b (IFN, a model protein drug) and albumin binding domain (ABD, a half-life extension module) are selected as the model proteins for bioconjugation and proof-of-concept.By employing SpyTag-SpyCatcher reaction and split-intein-mediated protein splicing, the biosynthesis of three protein topological isoforms with extremely similar amino acid compositions and sequences but distinct chemical topologies has been achieved directly in Escherichia coli. In the tadpole-shaped IFN-ABD conjugate (tadp-IFN-ABD), the SpyTag located within the sequence forms an isopeptide bond with the N-terminal SpyCatcher, resulting in the cyclization of IFN to enhance its stability, while ABD is positioned at the C-terminus to prolong circulation time. The figure-of-eight-shaped IFN-ABD conjugate(f8-IFN-ABD) has been constructed by further covalently conjugating the C-terminus of ABD with the N-terminus of SpyCatcher through split-intein-mediated ligation. By mutating the reaction site of SpyTag in f8-IFN-ABD, a macrocyclic IFN-ABD conjugate (c-IFN-ABD) has been produced, which only forms a non-covalent Spy complex with the same folded structure.Despite sharing the same constituent motifs, these three constructs possess different extents of conformational restriction due to distinct cyclization regions. Orthogonal proteolytic digestion experiments confirm the topological differences among the tadpole-shaped, macrocyclic, and figure-of-eight-shaped IFN-ABD conjugates.
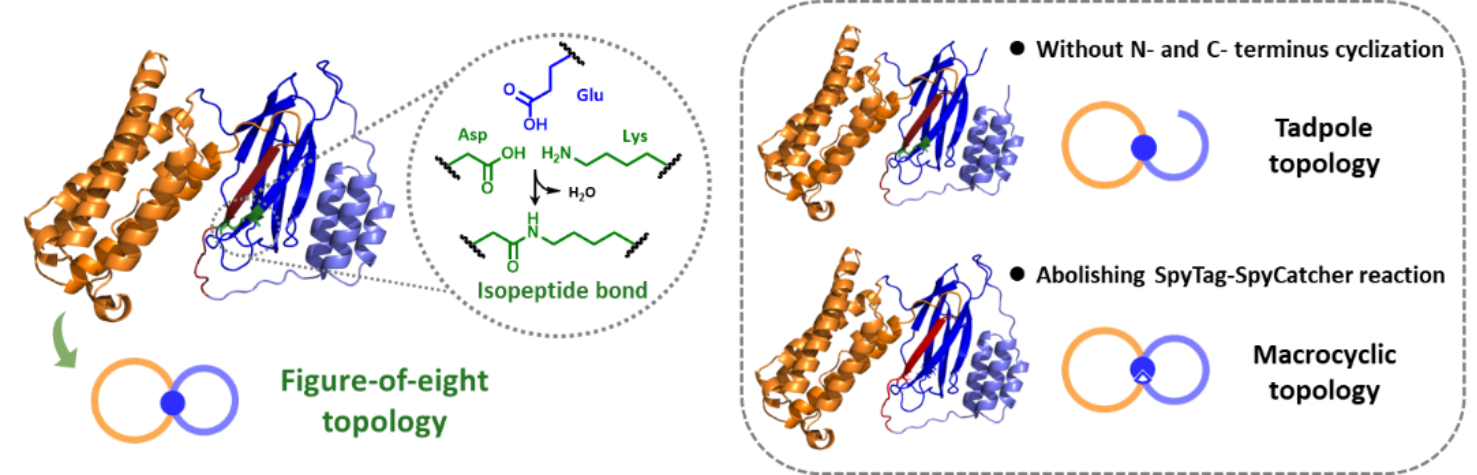
Fig. 1 Schematic illustration of the three IFN-ABD conjugates: figure-of-eight, tadpole, and macrocycle.
This work reveals that under non-denaturing conditions, the non-covalent complex formed by SpyTag and SpyCatcher exhibits similar structures to their covalent counterparts, making it challenging to distinguish topological differences between the macrocyclic and figure-of-eight-shaped IFN-ABD conjugates through various characterization methods, including CD spectroscopy, size exclusion chromatography, receptor affinity, and even half-maximal inhibitory concentration (IC50). There are significant differences in properties during the unfolding of the non-covalent complex. For instance, the f8-IFN-ABD conjugate demonstrated a higher melting temperature and better anti-aggregation ability compared to c-IFN-ABD, which is particularly beneficial for maintaining the structural integrity and biological activity of protein drugs during long-term storage.Interestingly, the tadpole-shaped IFN-ABD conjugate demonstrates slightly better thermal stability than c-IFN-ABD due to the presence of the covalent Spycomplex but was inferior to f8-IFN-ABD due to the uncyclized ABD moiety. These distinctions become more pronounced in animal experiments. In the pharmacokinetic assay,the measured elimination half-life of IFN is extended from ∼5.0 h to ∼20.4 h for tadp-IFN-ABD, ∼25.6 h for c-IFN-ABD, and ∼25.9 h for f8-IFN-ABD. Notably, the larger AUC of f8-IFN-ABD (52.6 μg·h·mL-1) compared to c-IFN-ABD (35.2 μg·h·mL-1) indicates improved absorption, distribution, and/or slower metabolism of f8-IFN-ABD.This would ultimately lead to higher levels of the protein drugs in the systemic circulation,demonstratingthat the presence of isopeptide bonds confers additional circulatory stability to the protein-based bioconjugate drugs. The f8-IFN-ABD conjugate also exhibits the most pronounced tumor accumulation in biodistribution experiments and outperforms the other two topological controls in terms of their antitumor activity in mouse tumor models.

Fig. 2 Investigation of topological effects among the three IFN-ABD conjugates. Thermal stability characterization and various animal experiments indicate that the figure-of-eight-shaped conjugate is the bestperforming candidate among the three topological isoforms.
The study summarizes the properties of the three topological protein isoforms to provide the following insights for maximizing the functional advantages of topology in practical applications: 1) both physical interactions and chemical crosslinking contribute to topological complexity and impose conformational restriction on the molecules.A higher number of valid bonding would imply a more pronounced conformational restriction and a higher stability; 2) cyclization would prolong the half-life of the bioconjugates, and the effect of cyclization would be affected by the ring size, where smaller ring sizes suggest a higher stability; 3) introducing covalent crosslinks into a well-folded structure could further promote the protein’s thermal stability as well as mechanical/proteolytic stability. By expanding beyond the linear paradigm of protein backbone, protein topology engineering is compatible with current manufacturing techniques and stabilization strategies, thus offering an additional dimension for fine-tuning the properties and performance of protein bioconjugates.
Dr. Yajie Liu (supported by the Boya Postdoctoral Fellowship) from the College of Chemistry and Molecular Engineering at Peking University isthe first author of this article.Professor Wen-Bin Zhang is the corresponding author. The work was supported by the National Key R&D Program of China, the National Natural Science Foundationof China, and the Beijing National Laboratory for Molecular Sciences.
Link to the original article:
//onlinelibrary.wiley.com/doi/full/10.1002/adhm.202400466